Clinical Research Data Warehouse
Need Data for Research or QI?
Access to data starts here! — Apply once, use often!
DATA REQUEST INTAKE FORM
IMPORTANT FORM COMPLETION INSTRUCTIONS
- Teams may apply for access prior to IRB or QI approval
- Teams may use de-identified query and data extract tools prior to approval
- There is no need to modify or resubmit a form once access is granted or approval is obtained
- Follow the eBridge SmartForm guidelines below for incorporating Honest Broker requirements
- Once IRB approval is obtained, identified patient data can be requested
- Please upload approval documents and/or data collection forms, if available, at the time of application
- Non-faculty requesting access must enter an MCW Faculty or Adjunct Faculty as the PI/Faculty Sponsor on the Project Team page
- Exception: Staff pharmacists and second year Pharmacy Residents (PGY2) have PI status
- Enter all other team members from CTSI partner institutions as well as any non-CTSI collaborators on the Project Team page
- Unfunded projects must use the FREE self-service Clinical Research Data Warehouse (CRDW) toolset unless re-routed by CTSI to another data provider
- Funded project teams should indicate their interest in using custom informatics services offered by the CTSI’s Center for Biomedical Informatics (CBMI)
CLINICAL RESEARCH DATA WAREHOUSE WORKFLOW
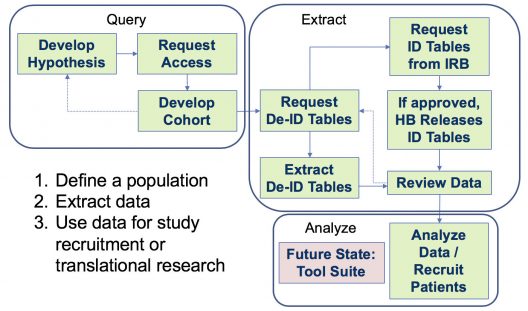
Special Features of CRDW Query Tools & De-Identified Honest Broker:
- You don’t need an approved IRB protocol to use our query tools.
- All data is de-identified.
- All dates are shifted.
- No names or IDs are available.
Requesting Identified Data from Honest Broker
- Receiving IRB approval does not guarantee identified Honest Broker approval
- Make sure your eBridge SmartForm is updated to include these Honest Broker checkpoints
- Contact cbmi-ri@mcw.edu to request a proactive protocol review if you are unsure how to amend your protocol
Honest Broker Checkpoints
Section 2.1 Project Team
- The person making the Honest Broker request must be listed in this section
- Adding team members is a quick administrative update to the protocol, no committee approval needed
Section 3.4 Minimal Risk Activities
- Check “Records Collected” since the CRDW is populated with EHR, billing, and other clinical source data
- This will open up Section 3F
Section 3F – Records Research
- 3F.2 & 3F.3 define the date range for your queries and extracts – Honest Broker date parameters must fall within these bounds
- 3F.4 Screen and Use – the screen total should be a reasonable overestimate and the use total should be some reasonable fraction of the screen number. In some cases it is okay for the Screen total to be double or triple the Use total.
Sections 17, 19 or 30 (Recruitment/Procedures)
- Depending on the type of project, there should be language about how the project might utilize the CRDW toolset
- Borrow template language linked here
- Be sure to mention both the query tools (i2b2 or TriNetX) and the Honest Broker process in your narrative
Section 26.2 Connecting with a Bank – accessing data
- Link to the CRDW banking protocol PRO00013874
DATA DOMAINS
The Honest Broker Data Dictionary contains detailed definitions for all our extractable tables.
2 MBCTSI Honest Broker Data Dictionary
The i2b2 cohort query and Honest Broker extract tools include the following data domains from which researchers can select criteria for cohort characterization and/or extract tables for analysis/recruitment.
- Allergies
- Biospecimens [Specimen Type, Tissue Site]
- COVID Concepts [Diagnoses, Lab Results & Procedures]
- Contact Information (for prospective recruitment)
- Demographics [Age, Email, Ethnicity, Language, Race, Sex, Vital Status]
- Diagnoses [ICD-9 & ICD-10]
- Diagnostic Results [Clinical Labs, Cardiovascular/Pulmonary Diagnostics, Radiation]
- Encounters (see Visit Details)
- Encounters – Future Appointments (for prospective recruitment)
- Genomics – genetic testing results from Foundation Medicine, Tempus, Invitae & Ambry
- Imaging Orders
- Immunizations
- Medications [Ordered/Administered]
- Pharmaceutical Class & Subclass
- Ingredient
- MAR Action, Route, Dose
- Ordering Mode
- NAACCR Tumor Registry from Froedtert Hospitals in Milwaukee, Menomonee Falls & West Bend
- OB/GYN Mom/Baby (delivery episode data)
- Problem List [ICD coding from Epic]
- Procedures [CPT, HCPCS and custom codes from Epic Orders]
- Procedures [CPT/HCPCS/ICD coding from Physician Billing & Hospital Billing]
- Providers (searchable by name and specialty)
- SDOH – Social Determinants of Health (data from the Epic SDOH Wheel)
- Social History/Lifestyle [Alcohol, Contraceptives, Illicit Drug Use, Tobacco Use]
- Surgical Cases
- Visit Details
- Age at Visit
- Department
- Visit Type (e.g., AV=ambulatory, IP=inpatient)
- Visit Vitals (outpatient only)
The TriNetX cohort query and analysis tool includes the following domains. Patient lists can be exported from TriNetX and used in Honest Broker to extract datasets.
- Demographics, Medications, Diagnoses, Labs, Procedures, Visits, and Genomics
Terms of Use
- This system was not designed, nor is it intended, to support any aspect of patient care and its use for any patient care purposes is prohibited.
- The results returned by this system may not be distributed outside of Medical College of Wisconsin.
- All searches and extracts executed within the system are recorded and will be examined, as part of routine compliance audits. The identity of the user is recorded along with information related to each search executed and each extract performed.
- No person registered as a user of the system may share their login information with any other person for purposes of accessing this system. Only registered users with Medical College of Wisconsin network credentials may use the system.
REQUIREMENTS
- CTSI membership
- Current MCW-required Human Subjects Research CITI Training
- Research Data Intake Form from the PI with all named research team members
- see Intake Form section above
- Medical College of Wisconsin network credentials (MCW username/password)
- MCW faculty or adjunct faculty can sponsor:
- CTSI academic faculty, postdocs, residents, fellows
- CTSI staff and students
- MCW faculty or adjunct faculty can sponsor:
- Access to the MCW network (MCW campus, remote VPN or mycitrix.mcw.edu)
LANGUAGE TO INCLUDE IN IRB APPLICATIONS
Read/Download Language to Include in IRB Applications
Citing the Clinical Research Data Warehouse
Investigators must cite both AHW and Medical College of Wisconsin CTSA Grant UL1TR001436.
This includes use of the self service cohort discovery tool, data consultations, clinical queries, and data extraction services made by our team.
AHW
“This project is funded in part by the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin.”
CTSA
Please acknowledge the Medical College of Wisconsin CTSA Grant UL1TR001436. All publications must comply with NIH Public Access Policy.
Researchers other than K Scholars (KL2) – Please cite Grant UL1TR001436, as follows:
“This publication [or project] was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001436. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.”
K Scholars (KL2) – Please cite Grant KL2TR001438, as follows:
“This publication [or project] was supported by the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health, through Grant Number KL2TR001438. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.”
Please see our citation information for details.
FEES / COST STRUCTURE NOTICE
Inquiries and consultations on new research projects are offered free of charge to CTSI community researchers. In general, Biomedical Informatics will provide 1-2 hours of labor for a project before we need to discuss setting up a payment plan.
When additional payment is required, we will execute a Statement of Work and Letter of Agreement with mutually agreeable terms. The PI will be responsible for covering a standard billing rate on an hourly basis for the time spent working on your project. Projects with larger scope or longer terms may be “contracted” via a Funding Proposal.
In projects where Biomedical Informatics expertise is required, we may engage in a collaborative partnership leading to co-authorship on related publications. In such a collaboration, and particularly where the work is of shared interest, we may be able to extend a cost-sharing of services.
BIOMEDICAL INFORMATICS PROJECT START-UP INCLUSIONS
Each project receives 1 hour of complimentary training and 1 hour of complimentary support. Biomedical Informatics support beyond the complimentary 2 hours, as outlined above, is considered out of scope and may affect your data delivery timeline and/or budget.
CUSTOM PROJECT SUPPORT
Contracted project support is billed hourly at a rate of $100 per hour. No project work can begin until a signed Letter of Agreement and Statement of Work has been fully executed. To achieve this state, the Biomedical Informatics team must receive a well-formed service request including but not limited to:
- project description,
- scope of work,
- cohort definition,
- comprehensive data element manifest with accompanying code sets,
- IRB approval documentation,
- external data use/sharing agreement(s),
- expected timeline,
- funding sources, and invoicing details.
SCOPE CHANGES
No work on scope changes will be done before both parties agree on cost and timeline changes and are client authorized with approval of a written change order.
Fees last updated June 30, 2017
ANSWERS TO FREQUENTLY ASKED QUESTIONS!
Do I need an IRB protocol to use the CRDW query tools?
No, the CRDW query tools ONLY contain fully de-identified data
Do I need an IRB protocol to use external data sources for my project?
Maybe. Please contact irboffice@mcw.edu to get this question answered
Currently, the use of SEER data does require teams to write an IRB protocol
Do I need an IRB protocol to extract identified data (real MRNs/real dates) from Honest Broker?
Yes, IRB approval is required and certain eBridge SmartForm sections must be populated. See requirements above!
Do I need an IRB protocol to publish findings for a project that uses CRDW data?
Maybe. Please contact irboffice@mcw.edu to get this question answered
Do I need an IRB protocol for a QI project that uses identified CRDW data?
You might… approval is required from the IRB or a designated QI authority in your department. If your department/division/center does not have a QA/QI review process, contact Froedtert’s OCRICC team or MCW’s IRB for advice on next steps.
DATA ANALYSIS
JUPYTER HUB
This self-service tool brings the power of a computational environment to researchers with advanced data science skills, allowing them to independently query and analyze encrypted Honest Broker tables directly.
TriNetX
This self-service research data platform is more than just a cohort discovery tool. TriNetX provides a variety of analytics features to complement their cohort development toolset. As well, teams can run queries and analyze data on larger research networks within the US and worldwide.
DATA MANAGEMENT TOOLS
The Center for Biomedical Informatics offers an instance of REDCap to support clinical research. We strongly encourage researchers to consult with a biostatistician from their department/division or the CTSI BERD team before beginning data analysis.
NIH Funding Acknowledgment: Important Reminder – Please acknowledge the NIH when publishing papers, patents, projects, and presentations resulting from the use of CTSI resources by including the NIH Funding Acknowledgement.